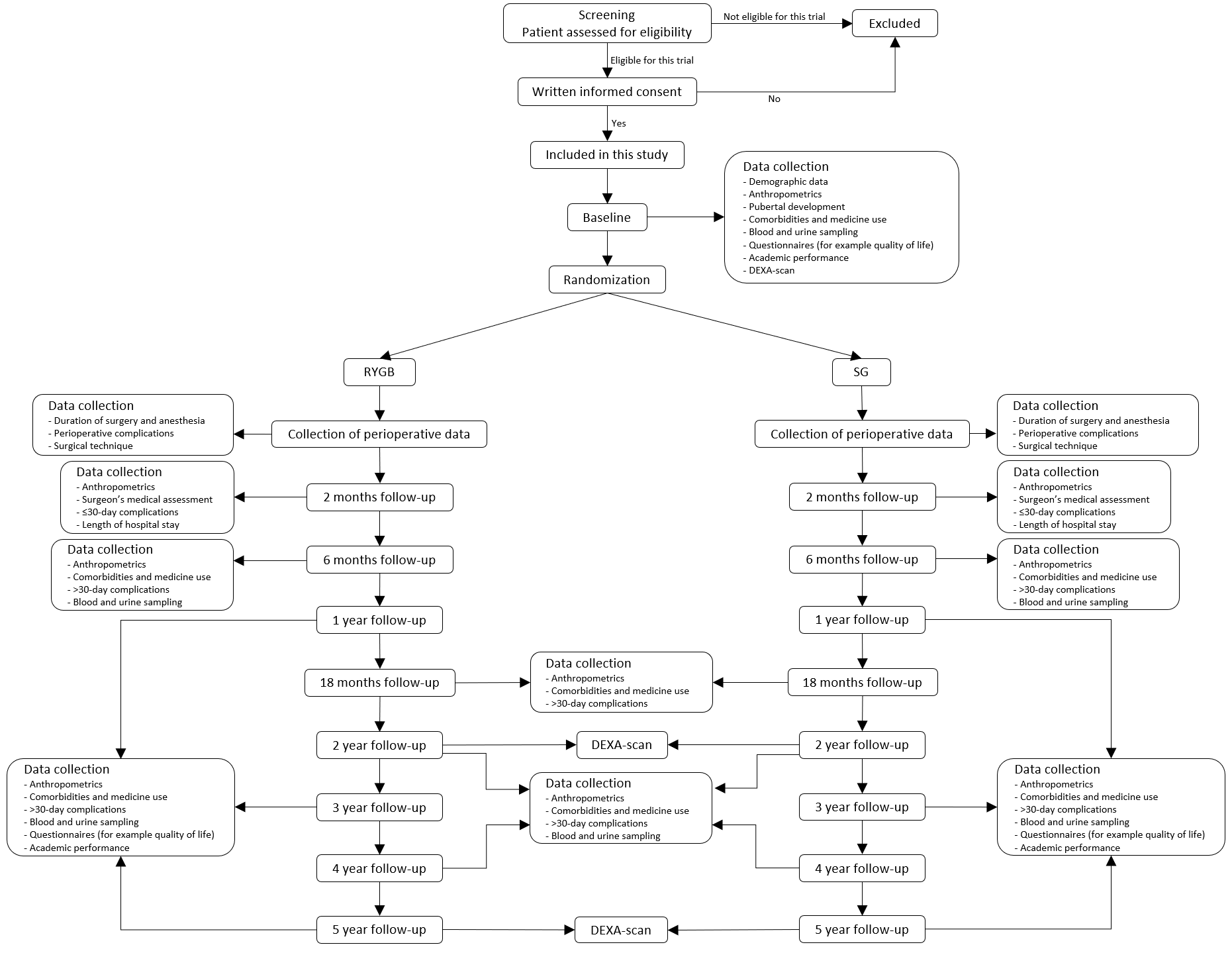
Method
Study Design
The TEEN-BESTrial is a partial blinded multicenter international randomized controlled non-inferiority trial. In total 264 adolescents will be included, 132 per arm (SG/RYGB). The follow-up will be five years.
A historical cohort of adolescents who participated in a lifestyle intervention program only (COACH – Maastricht UMC) will be used to compare both study arms with.
Outcomes
Primary outcome
Percentage total body weight loss after three years of follow-up.
Secondary outcomes
All secondary outcomes stated below are compared between SG and RYGB at one, three and five years after surgery. In addition, outcomes in these groups will also be compared with those of a historical cohort of adolescents who participated in a lifestyle intervention program (except for 2 and 7).
- Change in BMI and body weight;
- Incidence of adverse health events including the need for re-operation;
- Resolution of co-morbidities, including OSA, T2DM, hypertension and hyperlipidemia;
- Time to resolution of OSA, T2DM, hypertension and hyperlipidemia;
- Prevalence of cardio metabolic risk factor measures;
- Routine post-bariatric surgery nutritional blood tests at each assessment, including: full blood count, electrolytes, creatinine, fasting glucose, fasting insulin, HbA1c, liver parameters and function tests, iron, ferritin, vitamin B12, thiamine, folate/red cell folate, lipid profile, 25-hydroxyvitamin D, calcium and parathyroid hormone;
- Bone health measures;
- Generic and obesity-specific health-related quality of life;
- Psychosocial health measures and educational attainment;
- Patient satisfaction;
- Body composition.
Flowchart